Product Citations
Stapled, Long-Acting Glucagon-like Peptide 2 Analog with Efficacy in Dextran Sodium Sulfate Induced Mouse Colitis Models
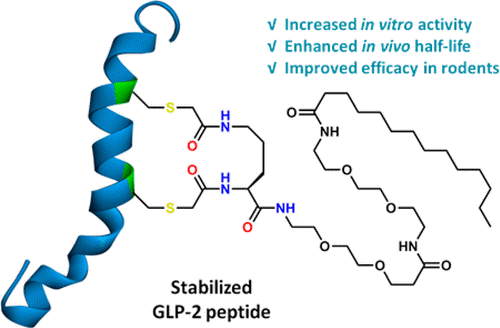 In this study, the authors have demonstrated that long-acting Glucagon-like peptide 2 (GLP-2) analogs can be generated by incorporating a serum protein binding motif into a Cys staple. These analogs retain full in vitro potency of GLP-2 and have extended half-life in rodents. One of the lead peptides, peptide 10, exhibited intestinotrophic and anti-inflammatory effects in DSS-induced intestinal inflammation in mice. When compared to teduglutide (GLP2-2G) in the DSS-induced chronic colitis models in mice, peptide 10 had improved efficacy with better intestinotrophic effects. These improvements in pharmacokinetics and pharmacodynamics suggest that our long acting GLP-2 analogs could have therapeutic potential for the treatment of SBS and IBD and other intestinal insufficiency diseases.
In this study, the authors have demonstrated that long-acting Glucagon-like peptide 2 (GLP-2) analogs can be generated by incorporating a serum protein binding motif into a Cys staple. These analogs retain full in vitro potency of GLP-2 and have extended half-life in rodents. One of the lead peptides, peptide 10, exhibited intestinotrophic and anti-inflammatory effects in DSS-induced intestinal inflammation in mice. When compared to teduglutide (GLP2-2G) in the DSS-induced chronic colitis models in mice, peptide 10 had improved efficacy with better intestinotrophic effects. These improvements in pharmacokinetics and pharmacodynamics suggest that our long acting GLP-2 analogs could have therapeutic potential for the treatment of SBS and IBD and other intestinal insufficiency diseases.
P.-Y. Yang, et al. (2018) J. Med. Chem. 61: 3218-3223.
Reduction of Circulating Cancer Cells and Metastases in Breast-Cancer Models by a Potent EphA2-Agonistic Peptide–Drug Conjugate
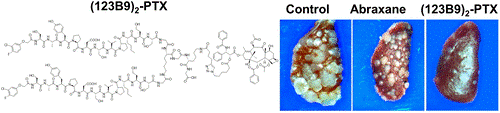 In this study, a dimeric version of 123B9 induced receptor activation at nanomolar concentrations. The conjugation of dimeric 123B9 with paclitaxel was very effective at targeting circulating tumor cells and inhibiting lung metastasis in breast-cancer models. These studies represent an important step toward the development of effective EphA2-targeting PDCs.
In this study, a dimeric version of 123B9 induced receptor activation at nanomolar concentrations. The conjugation of dimeric 123B9 with paclitaxel was very effective at targeting circulating tumor cells and inhibiting lung metastasis in breast-cancer models. These studies represent an important step toward the development of effective EphA2-targeting PDCs.
A.F. Salem, et al. (2018) J. Med. Chem. 61: 2052-2061.
A Helix-Stabilizing Linker Improves Subcutaneous Bioavailability of a Helical Peptide Independent of Linker Lipophilicity
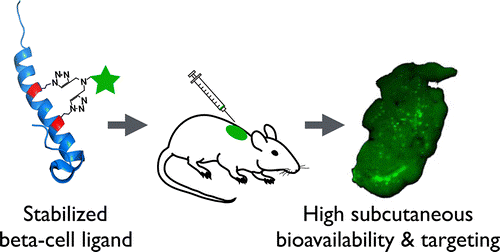 In this study, the authors investigated the effect of different fluorescent helix-stabilizing linkers with varying lipophilicity on subcutaneous (sc) bioavailability using the glucagon-like peptide-1 (GLP-1) receptor ligand exendin as a model system. The stabilized peptides showed significantly higher protease resistance and increased bioavailability independent of linker hydrophilicity, and all subcutaneously delivered conjugates were able to successfully target the islets of Langerhans with high specificity. The lipophilic peptide variants had slower absorption and plasma clearance than their respective hydrophilic conjugates, and the absolute bioavailability was also lower likely due to the longer residence times in the skin. Their ease and efficiency make double-click helix stabilization chemistries a useful tool for increasing the bioavailability of peptide therapeutics, many of which suffer from rapid in vivo protease degradation. Helix stabilization using linkers of varying lipophilicity can further control sc absorption and clearance rates to customize plasma pharmacokinetics.
In this study, the authors investigated the effect of different fluorescent helix-stabilizing linkers with varying lipophilicity on subcutaneous (sc) bioavailability using the glucagon-like peptide-1 (GLP-1) receptor ligand exendin as a model system. The stabilized peptides showed significantly higher protease resistance and increased bioavailability independent of linker hydrophilicity, and all subcutaneously delivered conjugates were able to successfully target the islets of Langerhans with high specificity. The lipophilic peptide variants had slower absorption and plasma clearance than their respective hydrophilic conjugates, and the absolute bioavailability was also lower likely due to the longer residence times in the skin. Their ease and efficiency make double-click helix stabilization chemistries a useful tool for increasing the bioavailability of peptide therapeutics, many of which suffer from rapid in vivo protease degradation. Helix stabilization using linkers of varying lipophilicity can further control sc absorption and clearance rates to customize plasma pharmacokinetics.
L. Zhang, T. Navaratna, and G. M. Thurber (2017) Bioconjugate Chem. 27: 1663-1672.
Engineering a long-acting, potent GLP-1 analog for microstructure-based transdermal delivery
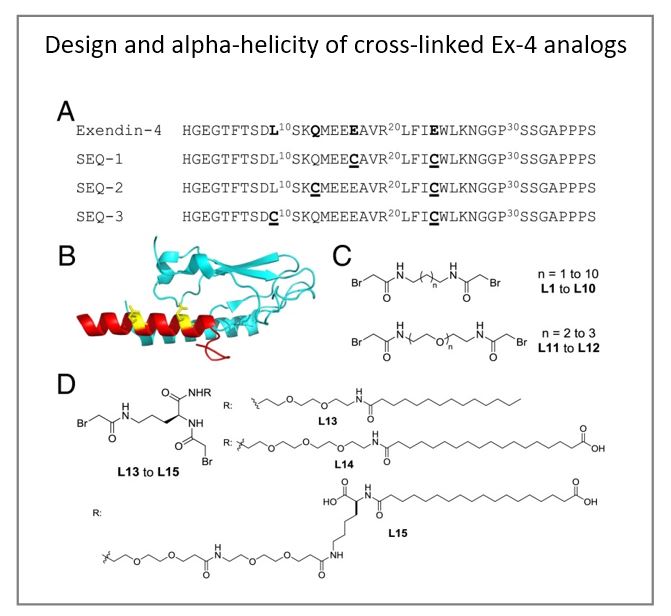 To further improve the therapeutic efficacy of exendin-4, the authors have developed a novel peptide engineering strategy that incorporates a serum protein binding motif onto a covalent side-chain staple and applied to the peptide to enhance its helicity and, as a consequence, its potency and serum half-life. The authors demonstrated that one of the resulting peptides, E6, has significantly improved half-life and glucose tolerance in an oral glucose tolerance test in rodents. Chronic treatment of E6 significantly decreased body weight and fasting blood glucose, improved lipid metabolism, and also reduced hepatic steatosis in diet-induced obese mice. Moreover, the high potency of E6 allowed us to administer this peptide using a dissolvable microstructure-based transdermal delivery system. Pharmacokinetic and pharmacodynamic studies in guinea pigs showed that a single 5-min application of a microstructure system containing E6 significantly improved glucose tolerance for 96 h. This delivery strategy may offer an effective and patient-friendly alternative to currently marketed GLP-1 injectables and can likely be extended to other peptide hormones.
To further improve the therapeutic efficacy of exendin-4, the authors have developed a novel peptide engineering strategy that incorporates a serum protein binding motif onto a covalent side-chain staple and applied to the peptide to enhance its helicity and, as a consequence, its potency and serum half-life. The authors demonstrated that one of the resulting peptides, E6, has significantly improved half-life and glucose tolerance in an oral glucose tolerance test in rodents. Chronic treatment of E6 significantly decreased body weight and fasting blood glucose, improved lipid metabolism, and also reduced hepatic steatosis in diet-induced obese mice. Moreover, the high potency of E6 allowed us to administer this peptide using a dissolvable microstructure-based transdermal delivery system. Pharmacokinetic and pharmacodynamic studies in guinea pigs showed that a single 5-min application of a microstructure system containing E6 significantly improved glucose tolerance for 96 h. This delivery strategy may offer an effective and patient-friendly alternative to currently marketed GLP-1 injectables and can likely be extended to other peptide hormones.
Engineering a long-acting, potent GLP-1 analog for microstructure-based transdermal delivery
P.-Y. Yang, et al. (2016) Proc Natl Acad Sci U S A. 113: 4140-4145.
hBfl-1/hNOXA Interaction Studies Provide New Insights on the Role of Bfl-1 in Cancer Cell Resistance and for the Design of Novel Anticancer Agents
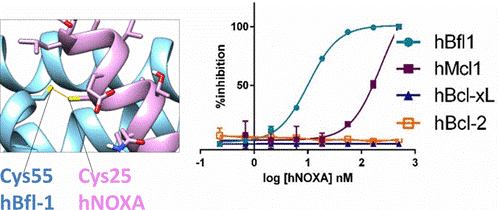 Recently, BH3 profiling was introduced as a method to classify cancer cells based on their ability to resist apoptosis following exposure to selected BH3 peptides. However, these studies were based on binding affinities measured with model BH3 peptides and Bcl-2-proteins taken from mouse sequences. While the majority of these interactions are conserved between mice and humans, the authors found surprisingly that human NOXA binds to human Bfl-1 potently and covalently via conserved Cys residues, with over 2 orders of magnitude increased affinity over hMcl-1. The data suggest that some assumptions of the original BH3 profiling need to be revisited and that perhaps further targeting efforts should be redirected toward Bfl-1, for which no suitable specific inhibitors or pharmacological tools have been reported. In this regard, the authors also describe the initial design and characterizations of novel covalent BH3-based agents that potently target Bfl-1. These molecules could provide a novel platform on which to design effective Bfl-1 targeting therapeutics.
Recently, BH3 profiling was introduced as a method to classify cancer cells based on their ability to resist apoptosis following exposure to selected BH3 peptides. However, these studies were based on binding affinities measured with model BH3 peptides and Bcl-2-proteins taken from mouse sequences. While the majority of these interactions are conserved between mice and humans, the authors found surprisingly that human NOXA binds to human Bfl-1 potently and covalently via conserved Cys residues, with over 2 orders of magnitude increased affinity over hMcl-1. The data suggest that some assumptions of the original BH3 profiling need to be revisited and that perhaps further targeting efforts should be redirected toward Bfl-1, for which no suitable specific inhibitors or pharmacological tools have been reported. In this regard, the authors also describe the initial design and characterizations of novel covalent BH3-based agents that potently target Bfl-1. These molecules could provide a novel platform on which to design effective Bfl-1 targeting therapeutics.
E. Barile, et al. (2017) ACS Chem Biol. 12: 444-455.
Secretion modification region-derived peptide blocks exosome release and mediates cell cycle arrest in breast cancer cells
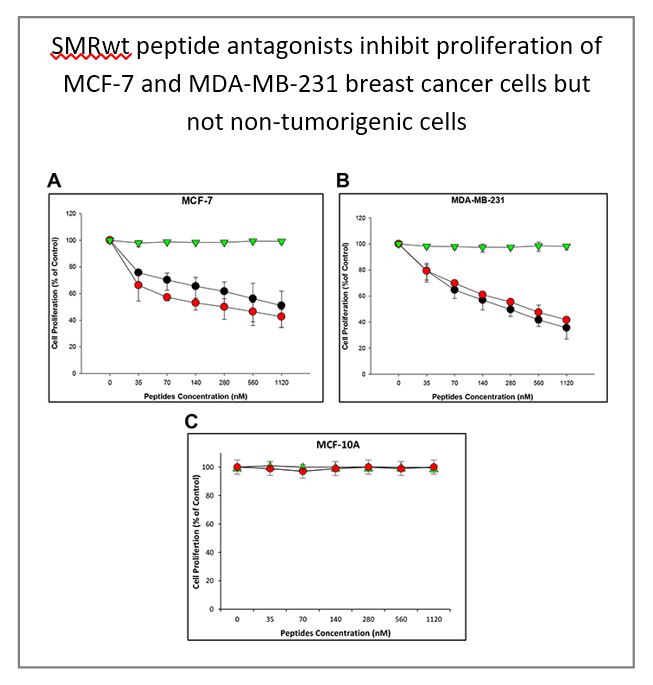
M.-B. Huang, et al. (2017) Oncotarget. 8: 11302-11315.
Dual-Purpose Linker for Alpha Helix Stabilization and Imaging Agent Conjugation to Glucagon-Like Peptide-1 Receptor Ligands
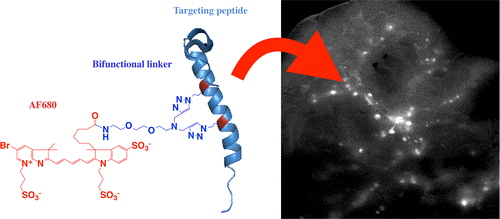 In this study, a new stabilized and labeled glucagon-like peptide-1 receptor (GLP-1R) ligand exendin showed an increase in helicity, improved protease resistance, negligible loss or an improvement in binding affinity, and excellent in vivo targeting. The ease of incorporating azidohomoalanine in peptides and efficient reaction with the dialkyne linker enable this technique to potentially be used as a general method for labeling α helices. This strategy should be useful for imaging beta cells in diabetes research and in developing and testing other peptide targeting agents.
In this study, a new stabilized and labeled glucagon-like peptide-1 receptor (GLP-1R) ligand exendin showed an increase in helicity, improved protease resistance, negligible loss or an improvement in binding affinity, and excellent in vivo targeting. The ease of incorporating azidohomoalanine in peptides and efficient reaction with the dialkyne linker enable this technique to potentially be used as a general method for labeling α helices. This strategy should be useful for imaging beta cells in diabetes research and in developing and testing other peptide targeting agents.
L. Zhang, T. Navaratna, J. Liang, and G. M. Thurber (2015) Bioconjugate Chem. 26: 329-337.
Design and Characterization of Novel EphA2 Agonists for Targeted Delivery of Chemotherapy to Cancer Cells
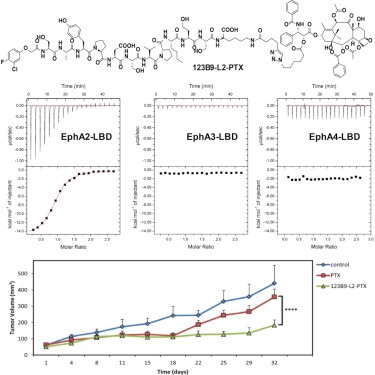
In this study, the author used a combination of nuclear magnetic resonance-guided structure-activity relationships along with biochemical and cellular studies to derive a novel tumor-homing agent, named 123B9, targeting the EphA2 tyrosine kinase receptor ligand-binding domain. Conjugating 123B9 to the chemotherapeutic drug paclitaxel (PTX) via a stable linker results in an agent that is significantly more effective than the unconjugated drug in both a pancreatic cancer xenograft model and a melanoma lung colonization and metastases model. Hence, 123B9 could represent a promising strategy for the development of novel targeted therapies for cancer.
B. Wu, et al. (2015) Cell Chem Biol. 22: 876-887.
Potent and Selective EphA4 Agonists for the Treatment of ALS
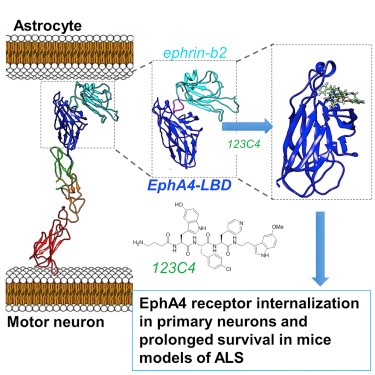 Recent studies identified the receptor tyrosine kinase EphA4 as a disease-modifying gene that is critical for the progression of motor neuron degeneration. In this study the authors report on the design and characterization of a family of EphA4 targeting agents that bind to its ligand binding domain with nanomolar affinity. The molecules exhibit excellent selectivity and display efficacy in a SOD1 mutant mouse model of ALS. Interestingly, the molecules appear to act as agonists for the receptor in certain surrogate cellular assays. While the exact mechanisms responsible for the therapeutic effect of the new agonists remain to be elucidated, the described agent represents both an invaluable pharmacological tool to further decipher the role of the EphA4 in ALS and potentially other human diseases, and a significant stepping stone for the development of novel treatments.
Recent studies identified the receptor tyrosine kinase EphA4 as a disease-modifying gene that is critical for the progression of motor neuron degeneration. In this study the authors report on the design and characterization of a family of EphA4 targeting agents that bind to its ligand binding domain with nanomolar affinity. The molecules exhibit excellent selectivity and display efficacy in a SOD1 mutant mouse model of ALS. Interestingly, the molecules appear to act as agonists for the receptor in certain surrogate cellular assays. While the exact mechanisms responsible for the therapeutic effect of the new agonists remain to be elucidated, the described agent represents both an invaluable pharmacological tool to further decipher the role of the EphA4 in ALS and potentially other human diseases, and a significant stepping stone for the development of novel treatments.
Potent and Selective EphA4 Agonists for the Treatment of ALS
B. Wu, et al. (2017) Cell Chem Biol. 24: 293-305.
