Services & Products
Custom Peptide Synthesis, Custom Antibody Service, Catalog Peptides.
Peptide Services
- Rush Peptide Service
- Custom Peptide Synthesis
- Peptide Library Service
- Peptide Conjugation Service
- Antibody Service
- Custom Chemical Synthesis
Custom Peptide Synthesis
Phosphorylated peptides; Citrullinated peptides; Succinylated peptides; Acetylated peptides; Amidated peptides; PEGylated peptides; Fluorescent peptides; Biotinylated peptides; Stable Isotope labeled peptides; Cyclic peptides; Stapled peptides; Branched Peptides; Metal Chelator peptides.
How it work?
- Peptide Amounts from mg to kg
- Peptide of 2-120 Amino Acids
- Purity of All Levels
- Comprehensive Modifications
- Peptide Conjugations
Rush Peptide Synthesis in 2-3 Days!
Please specify that your orders are for RUSH synthesis when ordering. Rush fee applies.
InnoPep provides the fastest turnaround time and most reliable quality for the life science research community. Peptides are made in San Diego, USA. Projects move from conception to bench in only 2–3 days so you can deal with your research deadlines.
Please specify that your orders are for RUSH synthesis when ordering. Our standard synthesis service, with its regular turnaround time of 1–2 weeks, is assumed unless the customer states otherwise.
Optional Local Pickup: Please contact us if you would like to pick up your peptides from our San Diego lab.

Peptides
- Phosphorylated peptides
- Citrullinated peptides
- Succinylated peptides
- Pegylated Peptides
- Fluorescent peptides
- Biotinylated peptides
- Cyclic Peptides
- Isotope Labeled Peptides
- Branched peptides
- Metal Chelator peptides
- Cyclization with multiple S-S bonds
- Stapled peptides
- Glycopeptides
- Histone Methylation Peptides
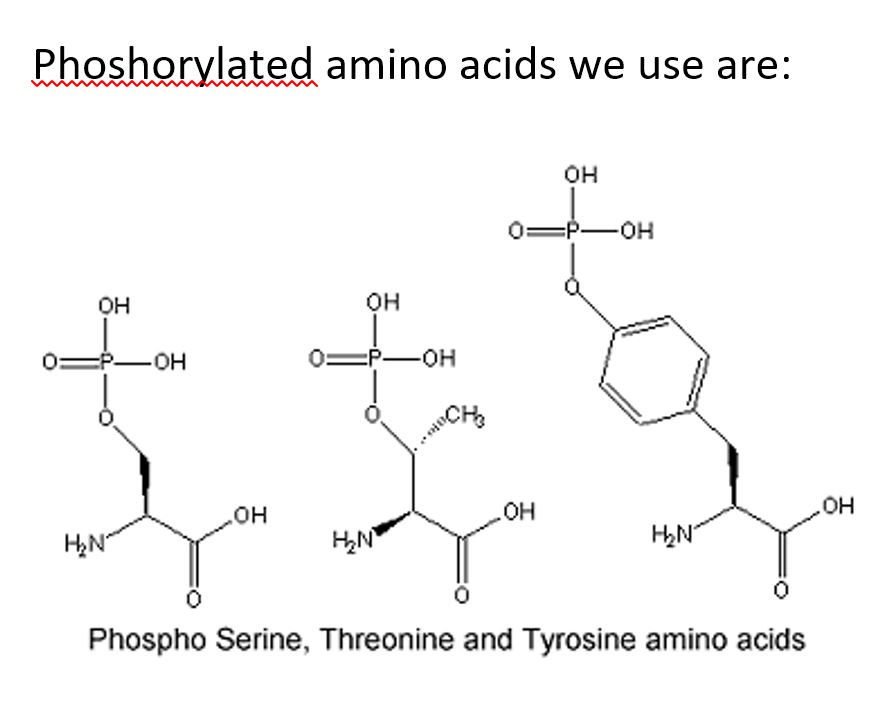
Peptide phosphorylation is a peptide containing phosphate (PO43-) group to a peptide containing serine, threonine or tyrosine. Phosphorylation is one of important post-translational modifications (PTMs) of protein function. Protein phosphorylation can occur on several amino acids.
Phospho-specific antibody generated by using phosphorylated peptide are commonly used to detect whether a protein is phosphorylated at a particular site. We offer peptide phosphorylation synthesis on pSer, pTyr, pThr or D-pSer, D-pTyr, D-pThr. We can also place phosphorylation site at multiple positions.
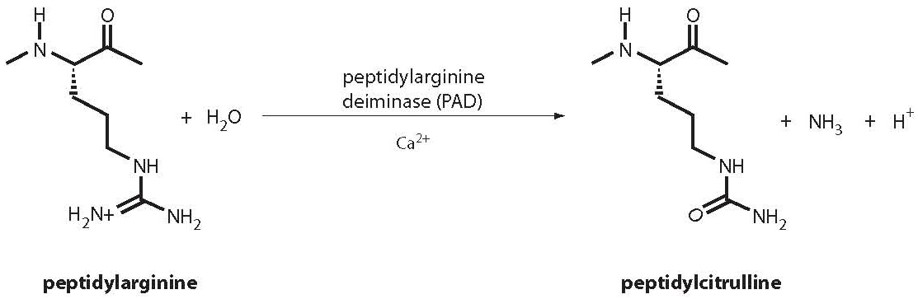
Citrullination is a post translational modification, where Arginine is deaminated to form Citrulline. The production of Citrulline containing peptides is a fairly straight forward process. They can be made at high purity, and we have provided quite a few of these over the years.
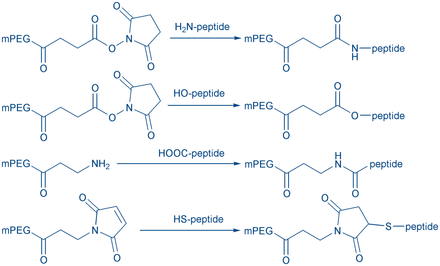
Peptide cyclization is a frequently used strategy for the development of peptides with enhanced conformational stability (compared to their linear analogs). Cyclic peptides metabolize slower due to their higher resistance toward proteases. They can be used to mimic the structure of biological active peptides (e.g peptide hormones).
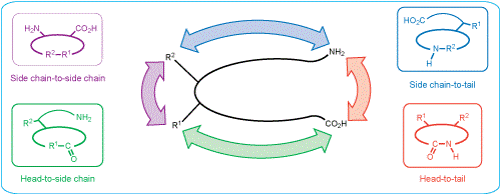
Depending on the cyclization site, there are several methods to synthesize cyclic peptides, e.g. head-to-tail, side-chain-to-side-chain, head-to-side-chain, and side-chain-to-tail (see figure below). While head-to-tail cycles are usually formed by amide bond formation, side-chain-to-side-chain cycles are most often synthesized via Cys-Cys or amide bond formation.
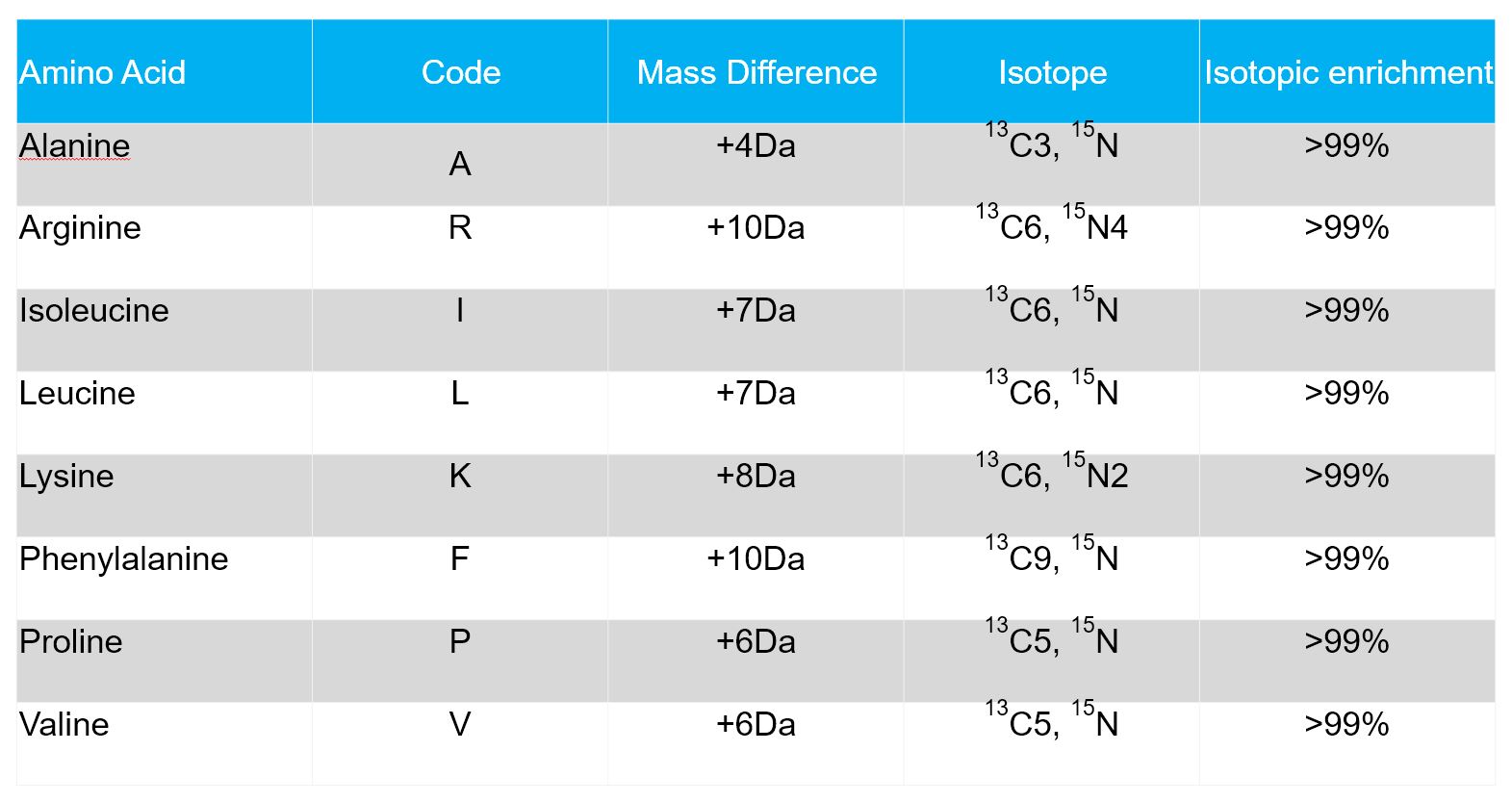
Isotope labeled or heavy amino acids are derived from natural amino acids by substitution of atoms with respective heavy isotopes. The most frequent substitutions of non-radioactive amino acids are 12C by 13C, of 14N by 15N, and of 1H by 2H (deuterium).
Isotope labeled peptides share the same physiochemical properties and (apart from a few exceptions) the same chemical reactivity with their non-labeled counterparts. However, under certain conditions labeled and unlabeled peptides behave differently. This is the basis for the use of stable isotope labeled peptides in a variety of applications, e.g.
- Reference material for mass spectrometry experiments (proteomics)
- NMR studies (e.g. solution structure determination)
- Reference material for pharmacokinetic analyses
- Metabolite identification
These stable isotopic heavy peptides are synthesized using the Fmoc solid-phase peptide technology in our state-of-the-art peptide lab. The table below lists the most common stable isotopically labeled amino acids.
Molecular imaging has become an indispensable tool in modern diagnostics in biomedical research and therapeutic fields. Modern imaging techniques increasingly rely on the use of radioactive isotopes of certain metal ions, often in combination with targeting peptides.
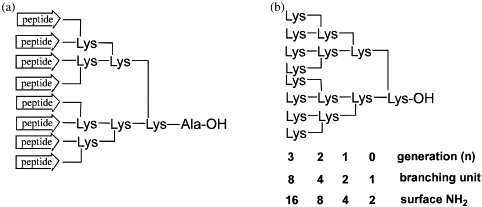
The modifications can be made at the N-terminus or at the C-terminus via a C-terminal lysine. These conjugates are able to give very stable complexes of many metals or with radioactive isotopes of metals currently used in Nuclear Medicine (In-111, Y-90,Tc-99m, Lu-177, Ga-68).
We routinely synthesize a variety of metal chelating peptides, such as DOTA-, DTPA-, and NOTA-coupled peptides.
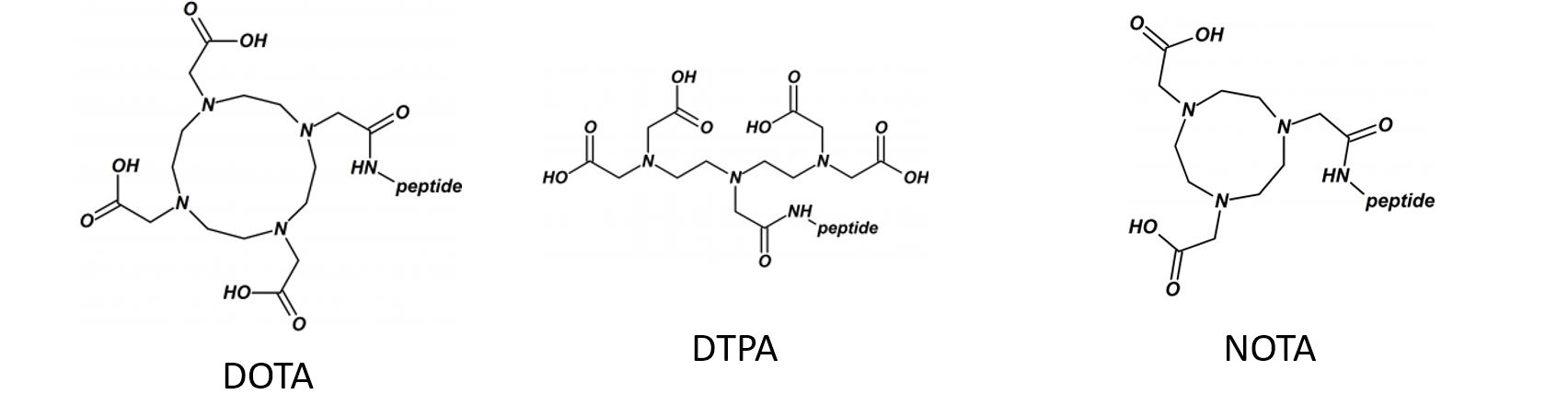
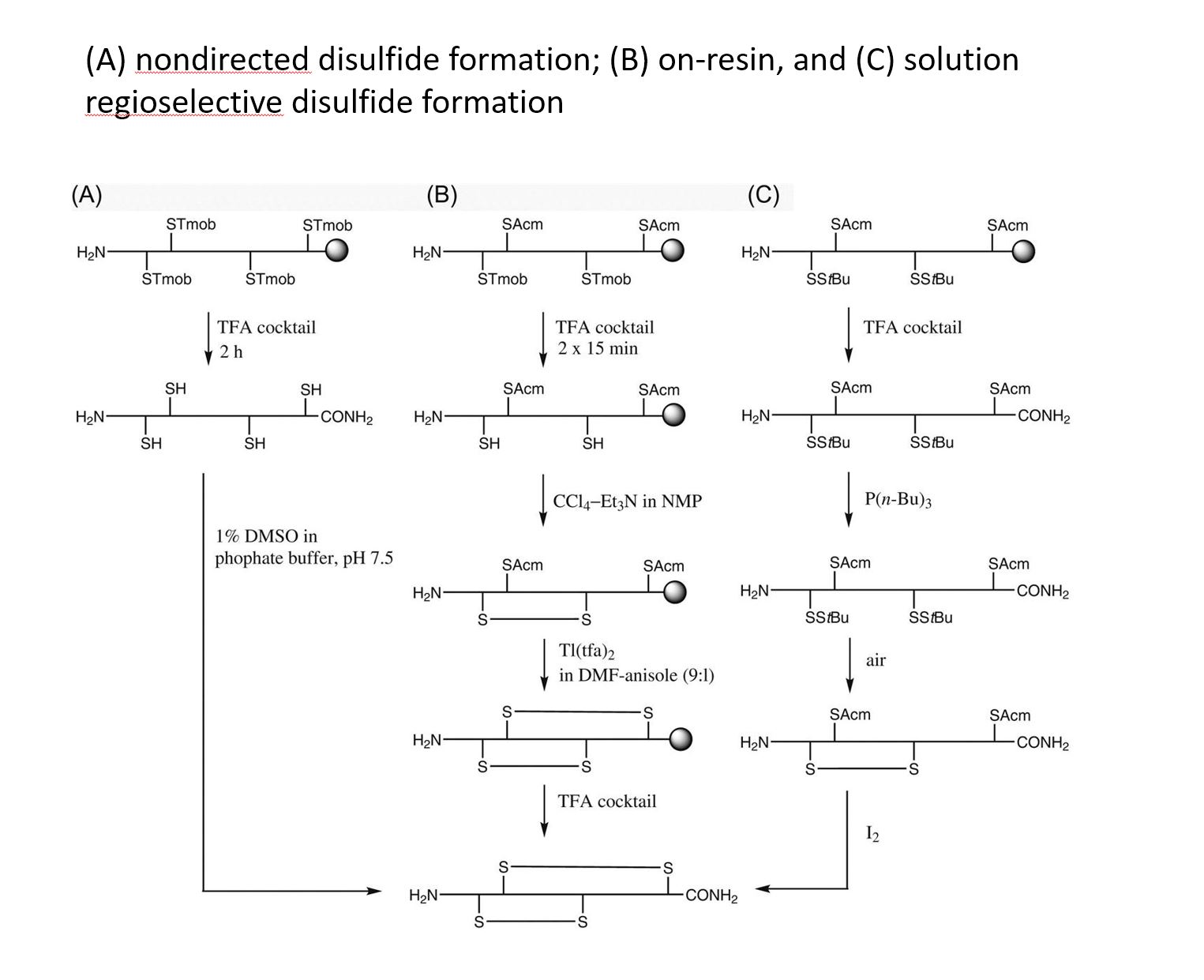
Disulfide-rich, bioactive macromolecules are widely represented in nature. Disulfide bonds stabilize higher-order structures that confer much enhanced chemical, biophysical, and metabolic stability. These higher-order structures are also integral to high potency and specificity in biological action, with conotoxins, epidermal growth factors, and the insulin superfamily peptides representing prominent examples.
Synthetic access to these peptides is essential to enable investigation of their structure−activity relationships and subsequent evaluation as drug candidates.
We applied and optimized the use of a series of different cysteine-protective groups (i.e. StBu, STmp, Trt, Mmt, Acm, tBu, etc.) that allow for a sequentially directed disulfide bond strategy (up to 4 different SS-bonds).
Stapled Peptides
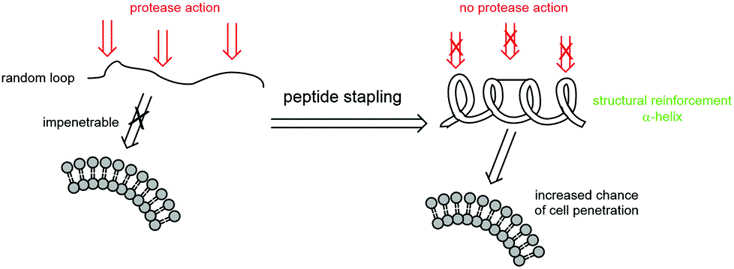
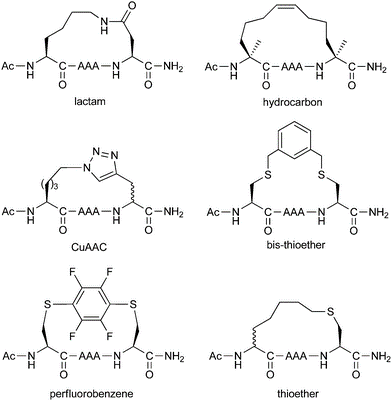
Different stapling chemistries have strengths and weaknesses in terms of both their synthetic ease and potential utility for addressing biological questions. To facilitate our clients’ research and project development, InnoPep is able to provide a comprehensive list of stapled peptides synthesis services, which includes: ring-closing metathesis, lactamization, click chemistry, and thioether formation.
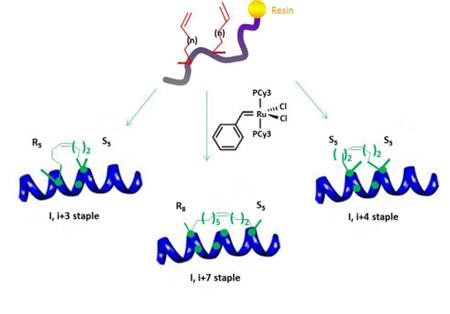
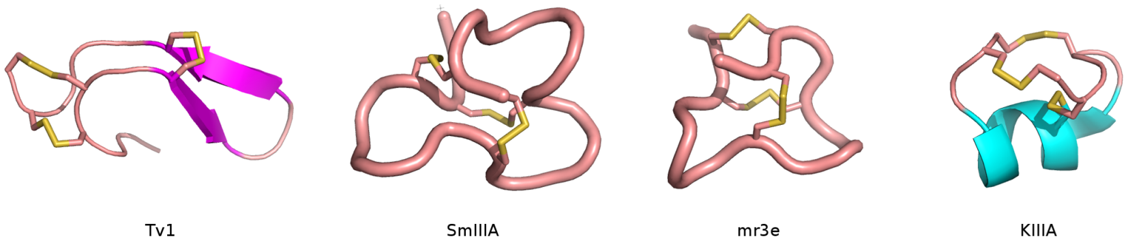
The all-hydrocarbon a-helix staple combines two distinct conformational stabilization strategies to induce a-helical structure, namely, a,a-disubstitution and macrocyclic bridge formation. Three different types of all hydrocarbon staples are shown in the figure, demonstrating the creation of a stabilized α-helix in a peptide. Approximately one turn of the helix would be i and i+3 (or i, i+4) and two turns of the helix would be i, i+7; three turns of the helix would be i, i+11. During solid-phase peptide synthesis, a-methyl, a-alkenylglycine cross-linking amino acids are incorporated in appropriate positions. An i, i+3 stapled peptide requires one unit of R5 at the i position and one unit of S5 at the i+3 position. An i, i+4 stapled peptide requires two units of S5 incorporated at the relative positions i and i+4. An i, i+7 stapled peptide requires one unit of R8 at the i position and one unit of S5 at the i+7 position. Resin-bound peptide is treated with Grubbs I olefin metathesis catalyst to produce a cross-link between the two non-natural amino acids, resulting in a stapled peptide that is braced in a a-helical conformation.
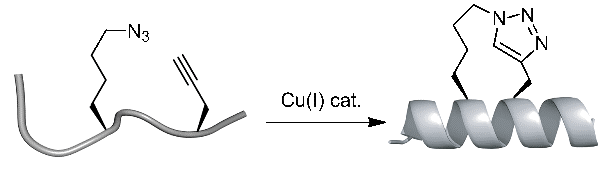 The high efficiency and mild conditions of “click” reaction (Copper catalyzed Huisgen 1,3-dipolar cycloaddition reaction) combined with the ease of synthesis of the necessary unnatural amino acids allows for facile synthesis of triazole-stapled peptides. For example, a combination of L-Nle(εN3) and D-Pra (D-Propargylalanine) substituted at the i and i+4 positions can be used for the generation of single triazole-stapled peptides.
The high efficiency and mild conditions of “click” reaction (Copper catalyzed Huisgen 1,3-dipolar cycloaddition reaction) combined with the ease of synthesis of the necessary unnatural amino acids allows for facile synthesis of triazole-stapled peptides. For example, a combination of L-Nle(εN3) and D-Pra (D-Propargylalanine) substituted at the i and i+4 positions can be used for the generation of single triazole-stapled peptides.
Advantages of Stapled Peptides
- Better target affinity
- Increased proteolytic resistance and serum half-life
- Cell penetration through endocytic vesicle trafficking
- Target either extracellular or intracellular proteins
- Disrupt Protein-Protein interactions
- Non-immunogenic
- Viable pharmacokinetics and in vivo stability
Stapled peptides have been studied in the targeting of several proteins which are relevant in Cancer, Diabetes, HIV, Atherosclerosis etc. These include proteins such as BCL-2, BCL-XL, BAX, MCL-1, glucokinase, hDM2, hDMX, NOTCH/CSL, HIV-1 capsid, HIV-1 gp41, ABCA1 and Estrogen receptor.
References
- Jackson DY, King DS, Chmielewski J, Singh S, & Schultz PG (1991) General approach to the synthesis of short alpha-helical peptides. J. Am. Chem. Soc. 113(24):9391-9392.
- Walensky LD, et al. (2004) Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 305(5689):1466-1470.
- Chang YS, et al. (2013) Stapled alpha-helical peptide drug development: A potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy. Proc. Natl. Acad. Sci. U. S. A. 110(36):E3445-E3454.
- Walensky LD & Bird GH (2014) Hydrocarbon-stapled peptides: principles, practice, and progress. J. Med. Chem. 57(15):6275-6288.
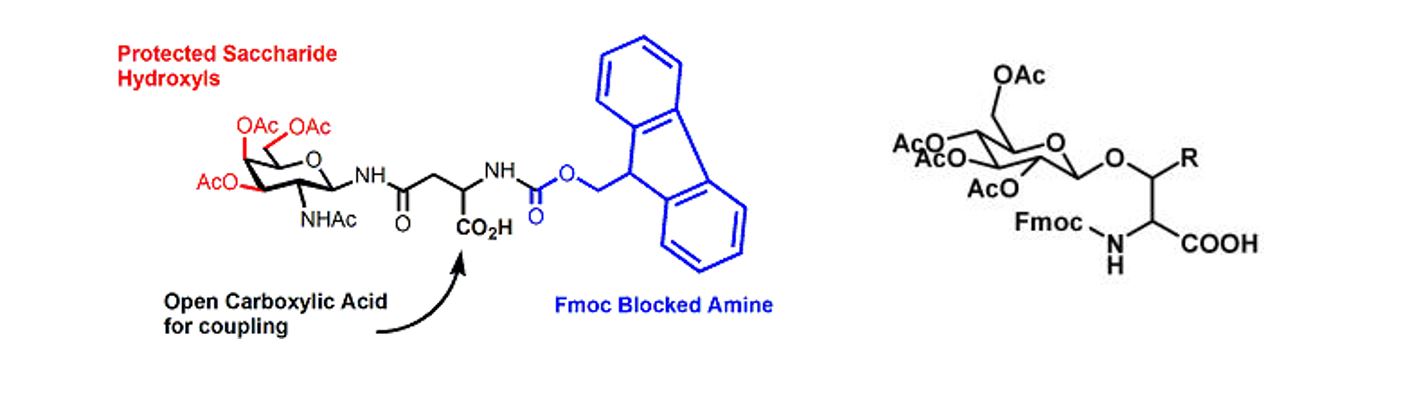
Glycopeptides, especially the glycan chains, have played pivotal roles in various biological activities and involved in numerous biological recognition events, such as immune system, endocrine system, protein folding and cell communication. Due to the natural linkage between glycan and the amino acid residues of peptide, glycopeptides can be classified into two principal types: N-linked and O-linked glycopeptides.
N-linked glycopeptides
- N-linked glycosylated peptides are the most frequently found glycopeptides
- They contain an amide bond between a glycan and the side chain of Asp, the so-called N-glycosidic bond
- The sites of N-glycosylation in N-glycoproteins are characterized by sequons Asn-Xaa-Ser/Thr (NXS/T, where Xaa is any amino acid except proline).
- The carbohydrate moiety is more variable, including alpha- and beta-Glc and beta-N-acetylgalactosamine (GalNAc)
O-linked glycopeptides
- O-linked glycoproteins are far more diversified than N-glycoproteins
- Most abundant is the mucin-type with an alpha-linkage between GalNAc and serine or threonine
- Examples of other saccharides linked to serine and threonine include alpha-galactose (Gal), glucose (Glc), fucose (Fuc), mannose (Man) and Xylose (Xyl)
- A separate class of O-linked glycosylation, which appears to be functionally distinct, is the O-GlcNAc modification found as monosaccharides attached to either Ser or Thr
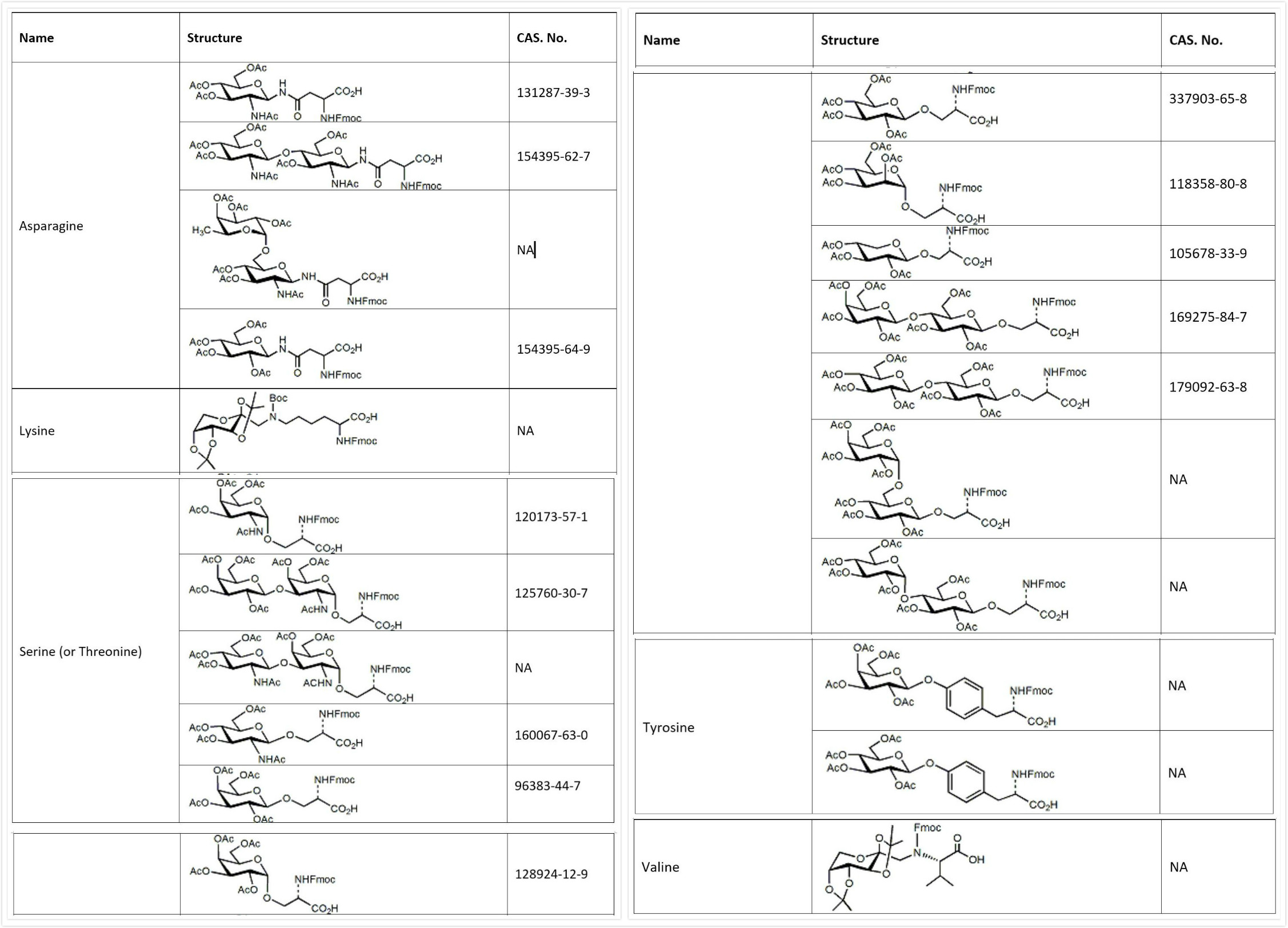
Glycoslyation of peptides has been an effective synthetic strategy to improve metabolic stability and enhance drug delivery. Glyco-amino acids used in solid phase peptide synthesis are Fmoc protected with the sugar generally protected as the fully acetylated form.
A selection of glycosylated amino acids that have been successfully incorporated into glycopeptides at InnoPep is shown below. Please contact us for your specific request.
References
- Rudd PM, et al. (2001) Glycosylation and the immune system. Science. 291(5512):2370-2376.
- Carganico S, et al. (2009) Building blocks for the synthesis of post-translationally modified glycated peptides and proteins. J. Pep. Sci. 15(2):67-71.
- Yamamoto T, et al. (2009) Improving metabolic stability by glycosylation: bifunctional peptide derivatives that are opioid receptor agonists and neurokinin 1 receptor antagonists. J. Med.
- Chem. 52(16):5164-5175.
Moradi SV, et al. (2016) Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem. Sci. 7(4):2492-2500.
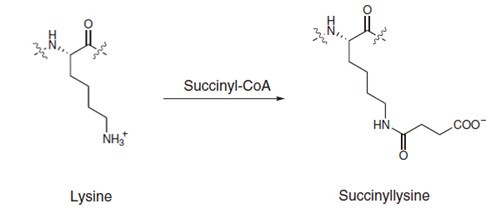
Proteins can be post-translationally modified in many different ways, and a common post-transcriptional modification of lysine involves methylation. Lysine can be methylated once, twice, or three times by lysine methyltransferases. The transfer of methyl groups from S-adenosyl methionine to histones is catalyzed by enzymes known as histone methyltransferases (HMTs).
Histone lysine methylation occurs on histones H3 and H4, and can signal either transcriptional activation or repression, depending on the sites of methylation. Methylation on the same site can also lead to different outcomes depending on the number of methyl groups added to lysine’s side chain. Histone methylation peptides of histone H3 and H4 include mono-methylated, di-methylated, and tri-methylated sequences. These epigenetic peptides are useful to researchers conducting studies in enzyme activity and screening inhibitors for specific HMTs.

With histone methylation playing critical roles in many epigenetic regulations, InnoPep is able to produce peptides with mono-, di- and tri-methylated Lys and Arg analogs listed.
References
- Martin C & Zhang Y (2005) The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 6(11):838-849.
- Chatterjee J, Rechenmacher F, & Kessler H (2013) N-methylation of peptides and proteins: an important element for modulating biological functions. Angew. Chem. Int. Ed. Engl. 52(1):254-269.
- Hamm CA & Coata FF (2015) Epigenomes as therapeutic targets. Pharmacol. Ther. 151:72-86.